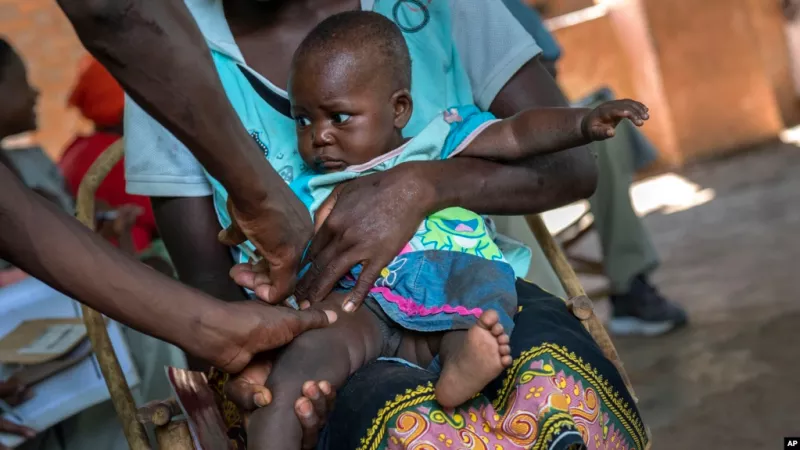
The World Health Organization (WHO) has announced the next step in the commercialization of the world's first approved malaria vaccine. The release is expected to take place in three African countries: Ghana, Kenya and Malawi.
But the Bill and Melinda Gates Foundation, a vaccine proponent, has raised concerns.
WHO said last fall that the vaccine was a "historic" development in efforts to combat malaria. But the Gates Foundation told the Associated Press this week that it will no longer financially support the vaccine.
Some scientists warn the decision could leave millions of African children at risk. It could also weaken future efforts to solve difficult problems in public health.
Read Also: Beautiful Self Portraits of Zanele Muholi: Dark Lioness
The vaccine is called Mosquirix and is sold by GlaxoSmithKline (GSK). It is about 30 percent effective and requires four doses.
The malaria vaccine has “a much lower efficacy than we would like,” said Philip Welkhoff. He is the Gates Foundation's director of malaria programs. The foundation is ending its support after spending more than $200 million and working more than 20 years to get the vaccine. Welkhoff also said the vaccine is too costly and difficult to provide.
Welkhoff said the foundation's money would be better spent on other malaria vaccines and treatments. Some of the resources that might have gone into providing the vaccine to countries have been redirected to buy new mosquito nets.
Alister Craig is with the Liverpool School of Tropical Medicine. “It’s not the greatest vaccine in the world, but there are ways of using it that could have a big impact,” he said.
The worldwide COVID-19 pandemic hurt efforts to stop malaria. Malaria killed more than 620,000 people in 2020 and caused 241 million cases, mainly in children under five in Africa, Craig said.
“There could be another vaccine approved in about five years, but that’s a lot of lives lost if we wait until then,” Craig said.
There is another vaccine currently being developed by Oxford University. BioNTech, creator of the Pfizer COVID-19 vaccine, also plans to use the messenger RNA technology it used for the coronavirus for a malaria shot. But that project has just begun.
Another big problem is production. GSK said it can only produce about 15 million injections per year until 2028. WHO estimates that to protect the 25 million children born in Africa every year, at least 100 million doses every year might be needed.
“All the money in the world” would not help the vaccine's short-term supply problems, said Welkhoff. He noted that the Gates Foundation continues to support the Gavi vaccine alliance. Gavi said it is spending nearly $156 million to make the vaccine available in the three African countries.
The vaccine, even with its problems, is still greatly desired in Malawi.
Nolia Zidana said she hopes to get her two young sons the shot after seeing malaria sicken them several times — and surviving it herself.
“Growing up with my parents and siblings, we have been sick from malaria all the time,” she said.
Dr. Michael Kayange is with Malawi's Ministry of Health. He urged everyone in the country to take whatever measures they can to stop malaria. Immunization itself is unable to stop the disease and people should take many measures, he said.
“Even just by sleeping under a mosquito net, you have played your role in reducing the malaria burden in the country,” he said.