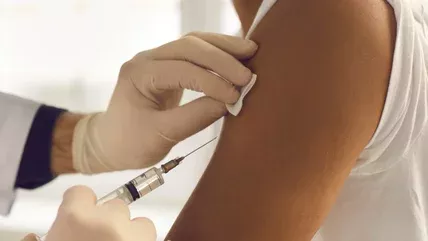
The Food and Drug Administration (FDA) recently approved a new COVID-19 boaster. According to a White House briefing, health experts hope (for those who qualify) it will become an annual vaccine to protect against COVID-19.
Read Also: CNBC - How ASML, TSMC And Intel Dominate The Chip Market
This latest COVID-19 booster is a combination, or bivalent, vaccine targeting the most common strains of the Omicron variant – BA.4 and BA.5. Half of the vaccine contains the medication that was put into use starting in December 2020, and the other half is made up of drugs that target these specific Omicron strains. The Pfizer-BioNTech booster is available to individuals 12 years old and up; the Moderna version is available for adults 18 years and older, per AP News.
Ideally, annual updated COVID-19 shots will provide enough protection for a full year against severe infection and illness for healthy individuals. Older adults and those with compromised immune systems may need more than a once-a-year shot. During the briefing, Director of the Centers for Disease Control and Prevention (CDC) Dr. Rochelle Walensky voiced her support for an annual COVID shot. She emphasized that preliminary data suggests updated COVID-19 vaccine doses this fall could prevent up to 100,000 hospitalizations and 9,000 deaths — as well as save billions of dollars in medical costs.
Should I get the COVID-19 booster and flu shot at the same time?
You might also be interested